Globally, over fifteen million people are living with spinal cord injury (SCI). Most SCI cases are due to trauma, including falls and road traffic injuries or violence. The term spinal cord injury (SCI) is used to describe the injury of the spinal cord caused by trauma or non-traumatic causes such as tumours, degenerative and vascular diseases, infections, toxins, or defects of birth.
Injury severity and location in the spinal cord figure out the amount of SCI-related impairment. SCI leads to complete or partial loss of sensory and/or motor functions below the level of injury. In paraplegia the arm functions are spared; in tetraplegia, they are affected. Autonomic nervous system dysfunction affecting different functions can be seen at any level of injury.
Currently, there is no way to reverse spinal cord damage. However, researchers are actively exploring new treatments, such as advanced prosthetics and medications that may help regenerate nerve cells or enhance the functioning of remaining nerves after an injury. While a complete cure is not yet available, ongoing efforts aim to find recovery solutions. Meanwhile, treatment primarily focuses on preventing additional harm and helping individuals adapt, regain independence, and lead fulfilling lives despite their injuries. This approach includes rehabilitation, therapy, and support systems to maximise quality of life.
Stem-cell therapy
Stem cell therapy is an advanced outpatient procedure that harnesses the body’s innate healing capabilities to repair damaged tissues, promote more efficient injury recovery, and alleviate pain. Unlike traditional methods that rely on medication or surgery, this treatment directly addresses the root cause of pain, enhancing function and mobility while offering a quicker recovery period. Stem cells can transform into bone, cartilage, or fat cells, aiding in the body’s natural healing process.
Transplanting stem cells or their early-stage counterparts could play a key role in repairing spinal cord injuries. Known for their remarkable ability to renew themselves and transform into any cell type in the body, stem cells have shown encouraging results in spinal cord injury research. Studies have found that these cells can be directed to become neurons or glial cells in the lab, offering hope for replacing the neural cells lost after such injuries.
Recently, Mayo Clinic researchers have shown the safety and potential benefits of stem cell regenerative medicine therapy for patients dealing with subacute and chronic spinal cord injuries. This breakthrough brings hope for enhanced treatment options for these complex, long-term conditions.
Stem Cell Trial Insights
At the Mayo Clinic, frontline research is shedding new light on the potential of stem cell therapy for spinal cord injuries. This research, which involves a diverse group of patients ranging from 18 to 65 years old, aims to explore whether stem cells can offer more than just hope.
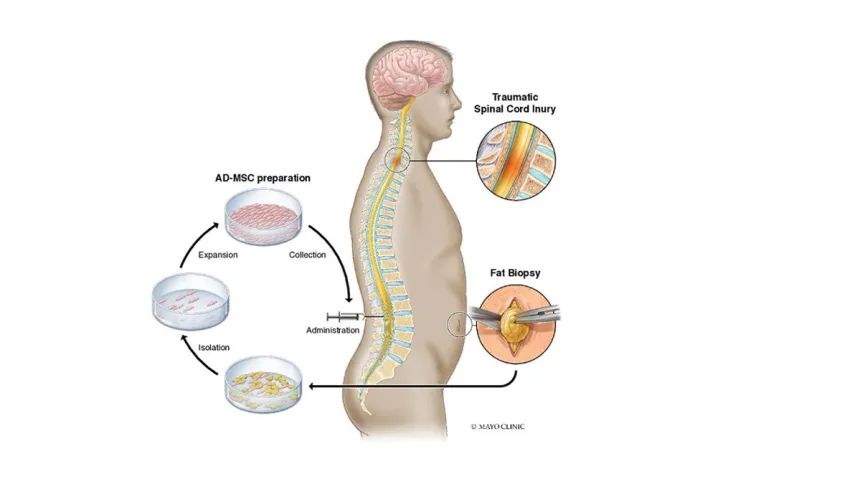
The participants, who suffered from spinal cord injuries due to accidents or falls, underwent a procedure where a small amount of fat was extracted through a minor incision in the abdomen or thigh. These fat-derived stem cells were then cultured in the lab over a month to grow to about 100 million cells. The cells were injected into the lower back, targeting the lumbar spine. Each participant was monitored at the Mayo Clinic ten times over two years to track their progress.
The science behind how stem cells interact with spinal cord injuries is still emerging. While it’s known that stem cells are drawn to inflamed areas, such as those affected by spinal cord injuries, their exact role in healing remains a mystery. Dr. Mohamad Bydon, a Mayo Clinic neurosurgeon and a leading researcher in the study, explains that the team is closely examining changes in MRIs and cerebrospinal fluid, along with evaluating pain responses and sensations. This detailed analysis aims to uncover how these cells might foster regeneration and healing at a cellular level.
Spinal cord injuries typically lead to the greatest recovery within the first six to twelve months, with improvements generally plateauing within one to two years. Notably, one participant with a neck injury who received stem cells 22 months after their injury experienced an improvement of one level on the ASIA scale, which measures motor and sensory function.
Also Read: American Hospital and Koç University Hospital partner with Mayo Clinic Care Network
The study also saw promising results in patients with complete thoracic spine injuries, where there was no feeling or movement below the injury site. Two out of three of these patients moved up two levels on the ASIA scale, regaining some sensation and control of movement. This is a significant achievement, given that traditionally, only 5% of those with complete thoracic injuries might expect such recovery.
Dr. Bydon notes that even a small improvement can drastically enhance a patient’s quality of life. Currently, stem cell treatments for spinal cord injuries are still considered experimental in the U.S., with fat-derived therapies classified as investigational by the Food and Drug Administration.
The next crucial step is to determine which patients benefit most from these therapies. A larger, controlled trial is underway, comparing stem cell treatment against a placebo. Dr. Bydon reflects on how traditional treatments for spinal cord injuries have focused mainly on stabilization and physical therapy, but this research offers a new direction. It challenges old assumptions and offers a promising path toward improving outcomes for those affected by spinal cord injuries.
New Hope in Therapy
Fat-derived stem cells were chosen for this study because of their accessibility and versatility. Adipose tissue is the richest source of mesenchymal stem cells, known specifically as adipose-derived mesenchymal stem cells (AD-MSCs). Their abundance and the ease of extraction make them an ideal option for research into spinal cord injuries. In this study, a small amount of fat was collected through a minor incision in the abdomen or thigh. Over the next four weeks, the stem cells were expanded in the lab to about 100 million cells and then injected into the patient’s lower back.
The study also saw promising results in patients with complete thoracic spine injuries, where there was no feeling or movement below the injury site.
The research team has also been looking at changes in MRI scans and cerebrospinal fluid to explore how these stem cells might connect to improvements in sensation and movement. While the Phase I trial didn’t provide enough data to link imaging changes directly to outcomes, this area is being investigated further in the ongoing Phase II trial and future studies.
One of the key findings from the first study is that there were no serious adverse events among participants. By the final follow-up, seven patients showed an improvement in their AIS grade, with two moving from a complete injury (Grade A) to a partial recovery (Grade C). This suggests that stem cell therapy might encourage recovery even beyond the typical 12 to 24-month window. The next steps involve deeper research into how these stem cells work and their long-term impact on spinal cord injury, with the Phase II trial already underway and more trials planned for the future.
Advances in spinal cord injury stem cell therapy are opening new avenues for patient recovery thanks to research conducted at the Mayo Clinic. The study found that the application of fat-derived stem cells improved mobility and feeling even after the typical recuperation period. These results stand for a significant advancement in therapy choices, even though they are still in the experimental stage. This distinctive approach may change the prognosis for people with spinal cord injuries as research advances, giving them new hope for an improved quality of life.