New test offers a less invasive, more accessible option to detect amyloid plaques associated with Alzheimer’s
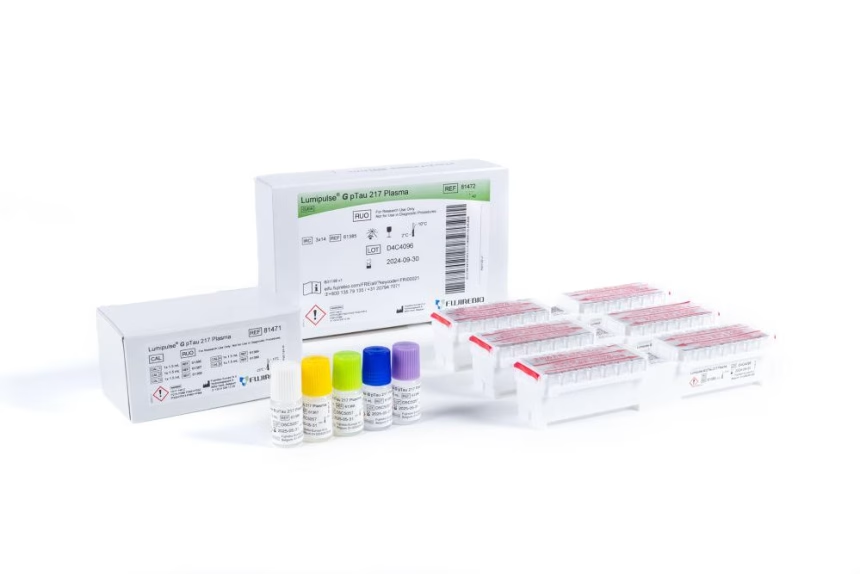
In a significant development for early Alzheimer’s disease detection, the U.S. Food and Drug Administration (FDA) has cleared the first-ever blood test to assist in diagnosing the condition. The newly approved Lumipulse G pTau217/ß-Amyloid 1-42 Plasma Ratio test offers a less invasive alternative to current methods like PET scans or spinal taps, marking a milestone in the fight against this debilitating disease.
The test is designed for adults aged 55 and older who show signs or symptoms of cognitive impairment. It works by analyzing levels of two specific proteins in the blood—pTau217 and β-amyloid 1-42—and determining their ratio. This measurement helps doctors assess whether amyloid plaques, a key indicator of Alzheimer’s disease, are present in the brain.
“Alzheimer’s disease impacts too many people, more than breast cancer and prostate cancer combined,” said FDA Commissioner Martin A. Makary, M.D., M.P.H. “Knowing that 10% of people aged 65 and older have Alzheimer’s, and that by 2050 that number is expected to double, I am hopeful that new medical products such as this one will help patients.”
Traditionally, amyloid plaques are detected through costly and time-consuming PET brain scans or via analysis of cerebrospinal fluid collected through spinal taps. The new Lumipulse blood test simplifies the process with just a standard blood draw—making Alzheimer’s assessment more accessible, especially for those in remote or underserved areas.
“Nearly 7 million Americans are living with Alzheimer’s disease and this number is projected to rise to nearly 13 million,” said Dr. Michelle Tarver, Director of the FDA’s Center for Devices and Radiological Health. “Today’s clearance is an important step for Alzheimer’s disease diagnosis, making it easier and potentially more accessible for U.S. patients earlier in the disease.”
In a clinical study involving 499 plasma samples, the test demonstrated high accuracy. Over 91% of people who tested positive with the Lumipulse blood test were confirmed to have amyloid plaques through PET scans or cerebrospinal fluid testing. Similarly, over 97% of those who tested negative had no amyloid plaques confirmed by other diagnostic methods.
While promising, the test is not without limitations. The FDA cautions that it should not be used as a screening tool or stand-alone diagnostic test. Instead, results must be interpreted alongside other clinical evaluations and tests. There are also risks of false positives or false negatives, which could lead to misdiagnosis, unnecessary treatments, or delays in receiving appropriate care.
The FDA reviewed the Lumipulse G pTau217/ß-Amyloid 1-42 Plasma Ratio through its 510(k) premarket notification pathway, confirming its substantial equivalence to a prior test that uses cerebrospinal fluid samples. The test was also granted Breakthrough Device designation, a program aimed at accelerating the review of innovative technologies for serious conditions.
The Lumipulse G pTau217/ß-Amyloid 1-42 Plasma Ratio is developed by Fujirebio Diagnostics, Inc.