In a groundbreaking medical milestone, the NHS in England has become the first health system worldwide to roll out a revolutionary “Trojan horse” therapy that could transform the lives of thousands of blood cancer patients. This innovative treatment, belantamab mafodotin, represents a new frontier in cancer care, offering hope where traditional therapies have fallen short.
A Life-Changing Journey: Paul’s Story
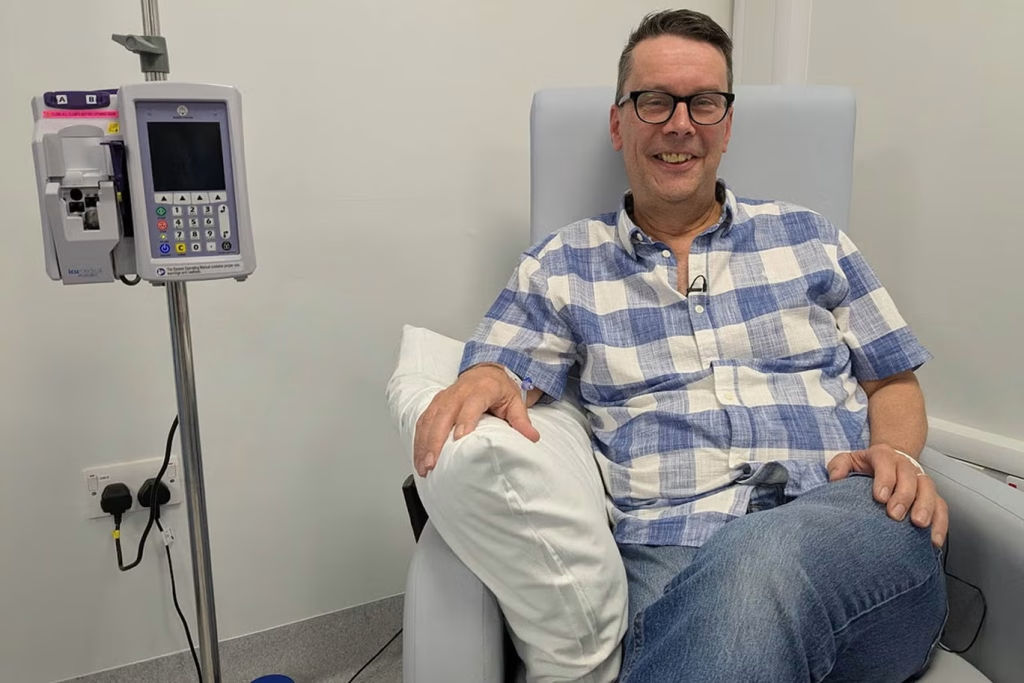
Paul Silvester, a 60-year-old history enthusiast from Sheffield, embodies the transformative power of this breakthrough therapy. Diagnosed with myeloma nearly two years ago after the cancer caused broken bones in his back, Paul’s journey illustrates both the devastating impact of this disease and the remarkable potential of targeted treatment.
After undergoing a bone marrow transplant that initially seemed successful, Paul faced the crushing reality of cancer relapse around Christmas. However, his participation in an early access program at the Royal Hallamshire Hospital in Sheffield would change everything.
“I feel like this treatment has brought the party balloons back in the house. It has been amazing — within the first two or three weeks, after the first dose, I was in remission,” Paul explains. “It gives me quite a lot of confidence in the drugs and it makes me more optimistic about the future. I’ve been feeling well and I’m still quite active — that’s what’s important in terms of your quality of life. One of my daughters is graduating from university in October and it’s a goal for me to be there.”
Within weeks of starting the new therapy, Paul was planning history-themed adventures and looking forward to his daughter’s university graduation.
Understanding the “Trojan Horse” Mechanism
Belantamab mafodotin represents a sophisticated evolution in cancer treatment, classified as an antibody-drug conjugate. The therapy earned its “Trojan horse” moniker from the ancient Greek tale of the siege of Troy, where soldiers were smuggled into the city within a wooden horse.
Similarly, this treatment works by binding a lethal chemotherapy drug to specially designed antibodies that recognize specific markings on plasma cells. These antibodies travel directly to cancerous cells, attach to their surface, and are absorbed. Once inside, they release their toxic payload, destroying the cancer from within.
Prof Martin Kaiser, team leader in myeloma molecular therapy at the Institute of Cancer Research, explains: “These are very smart drugs, and the difference in side effects compared to other drugs is really remarkable.”
Clinical Evidence and Impact
The clinical trial results are nothing short of remarkable. In trials involving patients with relapsed or refractory multiple myeloma, belantamab mafodotin (combined with bortezomib and dexamethasone) delayed disease progression by an average of three years, compared to just over a year for patients receiving commonly used drug daratumumab with the same combination.
This represents nearly a threefold improvement in progression-free survival, a metric that Professor Peter Johnson, NHS England’s National Clinical Director for Cancer, describes as having “the potential to keep cancer at bay for years longer, giving people the chance of more precious time with friends and family.”
“Myeloma is an aggressive type of blood cancer, but we have seen a steady improvement in the outlook for patients over recent years as we have introduced new targeted therapies,” Prof Johnson stated. “I am delighted that patients in England will be the first to benefit from this new treatment, which has the potential to keep cancer at bay for years longer, giving people the chance of more precious time with friends and family. This treatment could be life-changing for many patients and their families, and that’s why it is so important that the NHS continues to secure quick access to the latest, innovative treatments like this, at affordable prices to the taxpayer.”
The Myeloma Challenge
Multiple myeloma affects approximately 33,000 people living in the UK, with more than 6,000 new diagnoses each year. This aggressive blood cancer targets plasma cells within the bone marrow and often affects multiple body parts, including the spine, skull, pelvis, and ribs.
The disease disproportionately affects men over 60 and is twice as common in Black populations compared to white and Asian populations. In the Gulf Cooperation Council (GCC) region, the UAE and Qatar have reported the highest increases in MM cases across the world in the last three decades. The Middle East region has experienced a notable increase in multiple myeloma incidence, making access to innovative treatments particularly crucial for the region’s growing population of over 56 million people.
While myeloma remains incurable, the new therapy offers unprecedented hope for extended remission periods. Around 1,500 patients annually are expected to benefit from this treatment, specifically those whose cancer has progressed or failed to respond to first-line treatment with lenalidomide.
UK Innovation Leadership
This breakthrough showcases the UK’s position at the forefront of medical innovation. Belantamab mafodotin was partly discovered in Stevenage, with the first clinical trials conducted in London. The drug was developed by GSK in the UK, with early research taking place in Stevenage.
Antoine Herbaux, Vice President, Head of Oncology UK at GSK, noted: “We are delighted to have worked with NHS England and NICE on this significant recommendation in helping support unmet need in multiple myeloma. Today’s announcement highlights an example of local innovation. Belantamab mafodotin was partly discovered in Stevenage, the first patient to receive it in clinical trials was in London, and now the UK is the first country to grant patient access. This milestone is a great example of the power of scientific innovation and open collaboration to achieve positive outcomes for patients in the UK.”
Treatment Protocol and Considerations
The therapy is administered via infusion every three weeks, combined with bortezomib (injection) and dexamethasone (oral medication). The treatment duration is approximately 30 minutes, making it significantly more convenient than traditional chemotherapy regimens.
While the therapy is considerably kinder than conventional cancer treatments, it’s not without side effects. After cancer cell destruction, residual chemotherapy drugs can leak into the body, potentially causing ocular toxicity, including dry eyes, blurred vision, difficulty seeing clearly, and light sensitivity. Patients require routine ophthalmological assessments before starting treatment and after each of the first three treatments.
Looking to the Future
Prof Kaiser is optimistic about the future of myeloma treatment, suggesting that drugs like belantamab mafodotin represent “an important step towards a functional cure.” He predicts that long-term remission rates will exceed 50% within the next five years.
The success of antibody-drug conjugates extends beyond myeloma, with similar treatments being developed for breast, stomach, and bowel cancers. The challenge lies in designing antibodies that can specifically target cancer cells while sparing healthy tissue.
Healthcare System Impact
Health Minister Karin Smyth emphasized the broader implications: “This groundbreaking therapy puts the NHS at the forefront of cancer innovation. By harnessing cutting-edge ‘trojan horse’ technology, we’re offering new hope to blood cancer patients across the country. We’re determined to back scientific breakthroughs that deliver real results for patients, bringing the latest treatments from the lab to those who need them most. It’s another example of how we’re building an NHS fit for the future, one that embraces medical innovation to transform patient care.”
The approval came after a comprehensive review by the National Institute for Health and Care Excellence (NICE), which concluded the drug was cost-effective for NHS use. NHS England has fast-tracked access through immediate funding via the Cancer Drugs Fund.
Shelagh McKinlay, Director of Research and Advocacy at Myeloma UK, expressed her enthusiasm: “It’s fantastic to see the UK at the forefront of myeloma treatment. NHS England has demonstrated that it is possible for myeloma patients to have world-first access to innovative drugs. We have been working very hard for the last year to get this treatment approved and we know it will transform the lives of thousands of people with myeloma.”
Conclusion
The introduction of belantamab mafodotin represents more than just another treatment option, it symbolizes a new era in precision medicine where treatments can be tailored to seek and destroy cancer cells with unprecedented accuracy. For patients like Paul Silvester, it means the difference between isolation and adventure, between despair and hope.
As Paul prepares for his visit to Hadrian’s Wall and his daughter’s graduation, his story serves as a powerful reminder of what’s possible when innovative science meets compassionate healthcare policy. The NHS’s pioneering adoption of this “Trojan horse” therapy not only positions the UK at the forefront of cancer treatment but also offers a beacon of hope for myeloma patients worldwide.
This breakthrough underscores the critical importance of continued investment in medical research and the value of healthcare systems that can rapidly adopt and deliver life-changing innovations to those who need them most.