May 12, 2025 – Honolulu
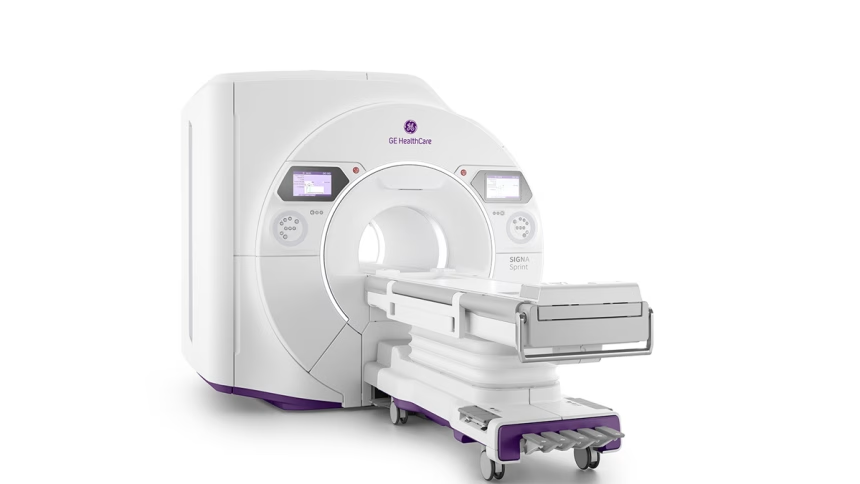
At the 2025 International Society for Magnetic Resonance in Medicine (ISMRM) meeting, GE HealthCare took center stage with the unveiling of its latest innovation: the SIGNA™ Sprint. This ultra-premium wide bore 1.5T MRI Imaging system is poised to disrupt the imaging landscape, bringing a level of power and clarity previously associated only with 3.0T systems.
With SIGNA Sprint, GE HealthCare is setting out to transform how clinicians diagnose and monitor cardiovascular and cancer patients, two areas where the demand for high-precision imaging is soaring. Cardiovascular disease and cancer remain among the world’s leading causes of death, making accurate, timely diagnostics critical.
What makes SIGNA Sprint remarkable is its high-performance gradient (65/200 per axis) – a leap forward in the 1.5T MRI category. This means faster scans and crystal-clear images, even when visualizing tiny anatomical structures. Designed with deep learning technology baked in, the system also promises to boost diagnostic confidence and efficiency, particularly in challenging imaging scenarios.
The scanner is equipped with built-in AI tools like AIR™ Recon DL, Sonic DL™, and AIR x™, which help deliver high-quality images while improving workflow and reducing patient scan time. These smart technologies aim to empower radiologists with greater consistency, especially when interpreting complex cardiac or oncological scans.
Beyond performance, GE HealthCare hasn’t forgotten the patient experience. SIGNA Sprint offers a roomy 70cm wide bore, free-breathing imaging capabilities, and its AIR™ Coils, which feel more like a soft blanket than traditional MRI gear, are all designed to reduce patient stress and improve overall comfort.
Kelly Londy, CEO of GE HealthCare’s MR division, said it best: “We’re pushing the limits of what’s possible in MRI. SIGNA Sprint is more than a scanner, it’s a strategic leap toward earlier detection, precision medicine, and next-level research capabilities.”
With the SIGNA Sprint currently pending FDA 510(k) clearance, the industry is watching closely as GE HealthCare continues to blur the lines between innovation, intelligence, and human-centered care.